SK Biopharmaceuticals launches anti-epileptic med in U.S.
SK Biopharmaceuticals launches anti-epileptic med in U.S.
Posted May. 13, 2020 07:31,
Updated May. 13, 2020 07:31
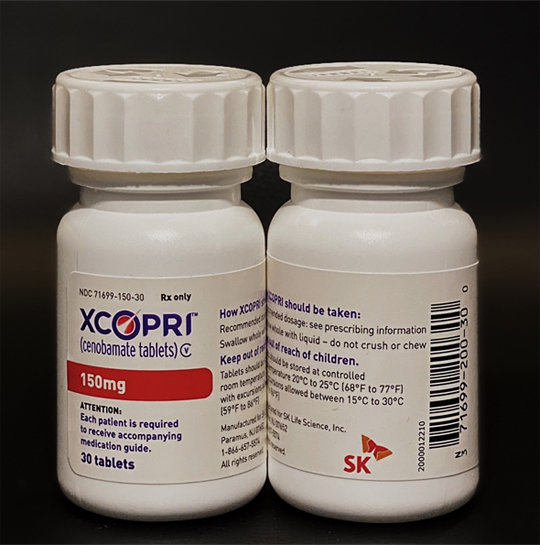
SK Biopharmaceuticals announced Tuesday the official launch of its anti-epileptic medicine cenobamate in the U.S. Cenobamate was independently developed by SK Biopharmaceuticals and was approved by the U.S. Food and Drug Administration (FDA) in November last year as an anti-epileptic drug for the treatment of partial-onset seizures in adults. It is the first time that a Korean pharmaceutical company directly applied for FDA’s approval without exporting technology for new medicine.
Local distribution is provided by SK Life Science, U.S. subsidiaryof SK Biopharmaceuticals. It will be distributed under the name “Xcopri.” According to business consulting firms including Frost & Sullivan, the U.S. epilepsy therapy market is valued at 3.3 billion dollars, which is projected to grow to 4.1 billion dollars by 2024.
"I congratulate the successful launch in the U.S. as Korea’s first FDA-approved and independently developed medicine,” said SK Group Chairman Chey Tae-won who consistently supported SK Biopharmaceutical’s development of new medicine. Cenobamate has applied for an approval of the European Medicines Agency.
warum@donga.com