Bio Tree: "Pay Attention to the Ecklonia Extract 'Seapolynol'"
Bio Tree: "Pay Attention to the Ecklonia Extract 'Seapolynol'"
Posted December. 30, 2024 10:56,
Updated December. 30, 2024 10:56
Seaweed varieties such as sea mustard, kelp, and laver, which are commonly consumed in Korea, are rich in nutrients like protein, vitamins, minerals, and dietary fiber. While seaweed is widely consumed in Korea and Japan as a "vegetable of the sea," other countries often perceive it as merely "seaweed." Among the varieties, Ecklonia cava is recognized for its benefits, such as dementia prevention, vascular health, and anti-cancer properties, earning it the title of a natural supplement from the sea. Recently, global interest has surged in the Ecklonia cava harvested from Korea’s coastal regions, particularly around Jeju Island.
A healthcare startup that has been developing and marketing products using the valuable nutrients extracted from Ecklonia cava for dietary supplements, pharmaceuticals, daily necessities, and cosmetics is steadily growing, both domestically and internationally. Bio Tree is the first company in Korea to introduce "Seapolynol" (a marine polyphenol extracted from Ecklonia cava), and has made a mark in the pharmaceutical and consumer goods market. We spoke with the Bio Tree Chairman Hyun-mo Shin, to learn more about the excellence of Korean Ecklonia cava and to discuss Bio Tree’s growth journey.
What is the difference between the Ecklonia cava used by Bio Tree and the seaweed that is commonly eaten?
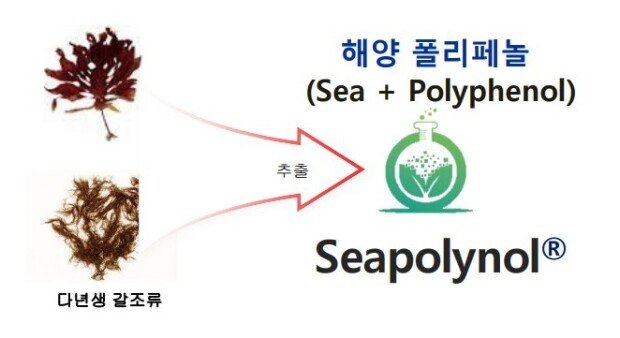
The seaweed commonly found on dining tables is officially named Ulva prolifera, which is a green alga. In contrast, the Ecklonia cava from which we extract polyphenols is a brown alga that resides at a depth of 15 meters (Ecklonia cava). The two forms of seaweed differ in their color, shape, and habitat. While the green Ecklonia cava is widely consumed, the brown Ecklonia cava is not palatable and is rarely eaten, even by fish. However, it is from this brown Ecklonia cava that we extract polyphenols and develop new drugs, dietary supplements, and daily necessities under our proprietary brand "Seapolynol."
It has been confirmed that the Ecklonia cava harvested from Korea, especially near Jeju Island, effectively removes radioactive substances from the body. Bio Tree has been the first company to extract 14 nutrients, including polyphenols, from Ecklonia cava for use in natural ingredients created for treating metabolic syndromes and diabetic complications. Clinical trials with the US FDA (Phase 1), Korea FDA (Phase 2a), and the FDA (Phase 2b) are currently underway.
 Marine polyphenol Seapolynol extracted from brown Ecklonia cava / Source: Bio Tree
Marine polyphenol Seapolynol extracted from brown Ecklonia cava / Source: Bio Tree
Bio Tree was founded in 2018, but Seapolynol received various certifications between 2008 and 2013. Can you explain the timeline leading to the company’s establishment?
Seapolynol (official name “Seapolynol CAF”) was initially developed as a new material called "Seanol" by BotaMedi, with international approvals being secured. BotaMedi is the precursor to Bio Tree. Key researchers and developers who were involved in its creation later established Bio Tree and continued their research and development activities. Notably, Dr. Ui-shin Kim, a world-renowned cancer treatment authority and a professor at the University of Texas MD Anderson Cancer Center, serves as Bio Tree's permanent advisor.
Since 2008, we have successively obtained US FDA NDI (New Dietary Ingredient) and IND (Investigational New Drug) certifications, as well as European EFSA NFI (Novel Food Ingredient) certifications, which is a first in Korea. We now hold over 50 patents or patent applications, and have published more than 130 SCI-grade papers.
As an entrepreneur with over 30 years of experience in education and distribution, what motivated you to enter the unfamiliar pharmaceutical and biotech startup industry?
As biotechnology gained recognition as the "second semiconductor," I became interested in its potential as a new growth driver. It felt like a necessary venture, and as someone who is at an age where concerns about diabetic complications and vascular diseases are significant, I decided to take on the challenge. The proven efficacy of Seapolynol, coupled with the market conditions and future growth potential, are what made the decision worthwhile.
While dietary supplements and drugs related to metabolic syndromes, diabetic complications, and vascular diseases exist, there are no products on the market that function as practical treatments or adjuvants with minimal side effects. This is why we are optimistic about the Seapolynol-based drugs currently undergoing clinical trials.
What is the focus of the current clinical tests?
Our primary focus is on "PH-100," a treatment for metabolic syndromes and diabetic complications using Seapolynol. The Phase 2b clinical trial is taking place in collaboration with 12 major hospitals in Korea, including four Catholic University hospitals, Korea University Anam Hospital, Kyung Hee University Hospital, Pusan National University Hospital, and others. Results from the Phase 2a clinical trials have shown a significant reduction in inflammatory markers (HS-CRP) and positive effects on cardiovascular diseases, without side effects. If the clinical trials proceed smoothly, the drug will be conditionally released on a prescription basis by the first half of 2026.
In addition to treatments, we have released "DangCut” (meaning to cut sugar), a functional food approved as a postprandial blood sugar improvement product. Beyond drugs, Bio Tree also produces and sells cosmetics and bath products made from Seapolynol (OEM orders).
 Brown Ecklonia cava used to extract Seapolynol / Source: Bio Tree
Brown Ecklonia cava used to extract Seapolynol / Source: Bio Tree
You are working on entering the Chinese market with the SBA’s support. What is your current export status?
We were invited by Jinan City in China’s Shandong Province to showcase our Seapolynol materials and key product lines. The local response was overwhelmingly positive, leading to exports of some of our products, such as liquid tea made from Seapolynol, which are now being sold in major Chinese cities, including Shanghai. Seapolynol’s versatility as a natural ingredient has also attracted interest from countries beyond China, including Japan, Hong Kong, Italy, the UK, the US, and the Middle East. In Hong Kong, a Bio Tree affiliate now operates as a branch office.
 Bio Tree participating in a corporate exchange meeting in Jinan, Shandong Province, China / Source: Bio Tree
Bio Tree participating in a corporate exchange meeting in Jinan, Shandong Province, China / Source: Bio Tree
What are the company’s sales and investment plans?
Currently, we are heavily reinvesting in our clinical research, with the combined sales from Bio Tree and its affiliates reaching approximately 2 billion KRW annually. We anticipate a significant increase in sales after PH-100 is officially launched in 2026.
Although we have received foreign investment and collaboration proposals, we’ve prioritized our independent operations in order to strengthen our corporate autonomy. However, as the clinical trials near completion, substantial funding will be required. From 2025 onward, we are planning to pursue investment opportunities more actively.
What are Bio Tree’s key plans for 2025?
While our clinical research has limited our participation in international conferences and exhibitions to date, we are planning to actively promote Seapolynol and our products on a global scale starting next year. We are aiming to collaborate with global companies in technology and business partnerships and will focus on export expansions. Domestically, we will renew our key products like DangCut, while broadening our brand recognition, and expanding our market reach.
After completing the clinical trials and launching the drug, we are planning to acquire domestic and international pharmaceutical companies to directly produce new drugs. An IPO and a listing on KOSDAQ are also under consideration. Our ultimate goal is to become a globally recognized pharmaceutical and healthcare company that represents Korea.
by Moon-gyu Lee (munch@itdonga.com)
A healthcare startup that has been developing and marketing products using the valuable nutrients extracted from Ecklonia cava for dietary supplements, pharmaceuticals, daily necessities, and cosmetics is steadily growing, both domestically and internationally. Bio Tree is the first company in Korea to introduce "Seapolynol" (a marine polyphenol extracted from Ecklonia cava), and has made a mark in the pharmaceutical and consumer goods market. We spoke with the Bio Tree Chairman Hyun-mo Shin, to learn more about the excellence of Korean Ecklonia cava and to discuss Bio Tree’s growth journey.
What is the difference between the Ecklonia cava used by Bio Tree and the seaweed that is commonly eaten?
The seaweed commonly found on dining tables is officially named Ulva prolifera, which is a green alga. In contrast, the Ecklonia cava from which we extract polyphenols is a brown alga that resides at a depth of 15 meters (Ecklonia cava). The two forms of seaweed differ in their color, shape, and habitat. While the green Ecklonia cava is widely consumed, the brown Ecklonia cava is not palatable and is rarely eaten, even by fish. However, it is from this brown Ecklonia cava that we extract polyphenols and develop new drugs, dietary supplements, and daily necessities under our proprietary brand "Seapolynol."
It has been confirmed that the Ecklonia cava harvested from Korea, especially near Jeju Island, effectively removes radioactive substances from the body. Bio Tree has been the first company to extract 14 nutrients, including polyphenols, from Ecklonia cava for use in natural ingredients created for treating metabolic syndromes and diabetic complications. Clinical trials with the US FDA (Phase 1), Korea FDA (Phase 2a), and the FDA (Phase 2b) are currently underway.

Bio Tree was founded in 2018, but Seapolynol received various certifications between 2008 and 2013. Can you explain the timeline leading to the company’s establishment?
Seapolynol (official name “Seapolynol CAF”) was initially developed as a new material called "Seanol" by BotaMedi, with international approvals being secured. BotaMedi is the precursor to Bio Tree. Key researchers and developers who were involved in its creation later established Bio Tree and continued their research and development activities. Notably, Dr. Ui-shin Kim, a world-renowned cancer treatment authority and a professor at the University of Texas MD Anderson Cancer Center, serves as Bio Tree's permanent advisor.
Since 2008, we have successively obtained US FDA NDI (New Dietary Ingredient) and IND (Investigational New Drug) certifications, as well as European EFSA NFI (Novel Food Ingredient) certifications, which is a first in Korea. We now hold over 50 patents or patent applications, and have published more than 130 SCI-grade papers.
As an entrepreneur with over 30 years of experience in education and distribution, what motivated you to enter the unfamiliar pharmaceutical and biotech startup industry?
As biotechnology gained recognition as the "second semiconductor," I became interested in its potential as a new growth driver. It felt like a necessary venture, and as someone who is at an age where concerns about diabetic complications and vascular diseases are significant, I decided to take on the challenge. The proven efficacy of Seapolynol, coupled with the market conditions and future growth potential, are what made the decision worthwhile.
While dietary supplements and drugs related to metabolic syndromes, diabetic complications, and vascular diseases exist, there are no products on the market that function as practical treatments or adjuvants with minimal side effects. This is why we are optimistic about the Seapolynol-based drugs currently undergoing clinical trials.
What is the focus of the current clinical tests?
Our primary focus is on "PH-100," a treatment for metabolic syndromes and diabetic complications using Seapolynol. The Phase 2b clinical trial is taking place in collaboration with 12 major hospitals in Korea, including four Catholic University hospitals, Korea University Anam Hospital, Kyung Hee University Hospital, Pusan National University Hospital, and others. Results from the Phase 2a clinical trials have shown a significant reduction in inflammatory markers (HS-CRP) and positive effects on cardiovascular diseases, without side effects. If the clinical trials proceed smoothly, the drug will be conditionally released on a prescription basis by the first half of 2026.
In addition to treatments, we have released "DangCut” (meaning to cut sugar), a functional food approved as a postprandial blood sugar improvement product. Beyond drugs, Bio Tree also produces and sells cosmetics and bath products made from Seapolynol (OEM orders).

You are working on entering the Chinese market with the SBA’s support. What is your current export status?
We were invited by Jinan City in China’s Shandong Province to showcase our Seapolynol materials and key product lines. The local response was overwhelmingly positive, leading to exports of some of our products, such as liquid tea made from Seapolynol, which are now being sold in major Chinese cities, including Shanghai. Seapolynol’s versatility as a natural ingredient has also attracted interest from countries beyond China, including Japan, Hong Kong, Italy, the UK, the US, and the Middle East. In Hong Kong, a Bio Tree affiliate now operates as a branch office.

What are the company’s sales and investment plans?
Currently, we are heavily reinvesting in our clinical research, with the combined sales from Bio Tree and its affiliates reaching approximately 2 billion KRW annually. We anticipate a significant increase in sales after PH-100 is officially launched in 2026.
Although we have received foreign investment and collaboration proposals, we’ve prioritized our independent operations in order to strengthen our corporate autonomy. However, as the clinical trials near completion, substantial funding will be required. From 2025 onward, we are planning to pursue investment opportunities more actively.
What are Bio Tree’s key plans for 2025?
While our clinical research has limited our participation in international conferences and exhibitions to date, we are planning to actively promote Seapolynol and our products on a global scale starting next year. We are aiming to collaborate with global companies in technology and business partnerships and will focus on export expansions. Domestically, we will renew our key products like DangCut, while broadening our brand recognition, and expanding our market reach.
After completing the clinical trials and launching the drug, we are planning to acquire domestic and international pharmaceutical companies to directly produce new drugs. An IPO and a listing on KOSDAQ are also under consideration. Our ultimate goal is to become a globally recognized pharmaceutical and healthcare company that represents Korea.
by Moon-gyu Lee (munch@itdonga.com)







